Why South Korean Biopharmaceutical Companies Need Scalable SaaS in 2025

The South Korean biopharmaceutical industry has seen exponential growth over the past decade, with increased R&D, patent filings, and international licensing deals. By 2025, the industry is expected to play a big role in promoting the country’s economy because of government support, a strong focus on research and development, and partnerships with international companies. However, one key factor that could either limit or increase this growth is the technology infrastructure, especially scalable Software-as-a-Service (SaaS) platforms.
In this article, we will look at the need for scalable SaaS platforms for South Korean biopharmaceutical businesses as well as the ways in which these technologies can promote competitiveness, global cooperation, innovation, and compliance.
South Korea is known as a leading hub for developments in biotechnology and pharmaceuticals. Prominent corporations like as Samsung Biologics, Celltrion, and LG Chem Life Sciences are making progress in fields including biologics manufacturing, vaccine manufacture, and biosimilars. The nation’s pharmaceutical industry is predicted to grow to $22.65 billion by 2025, propelled by expanding government funding, R&D expenditures, and a growing need for healthcare products.
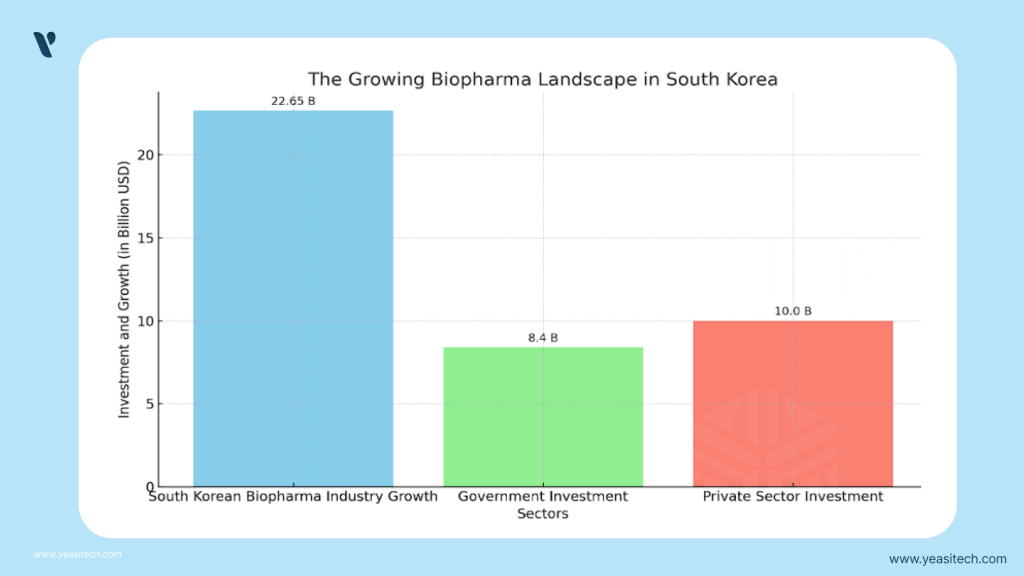
The government has contributed significantly to this growth’s funding. Policies like tax reductions for research and development, funding programs like the Ministry of Science and ICT’s Bio-Venture Support Program, and partnerships between the public and private sectors have all helped businesses—both new and established—to prosper.
The administration of President Moon Jae-in has committed to making significant investments in the biotech sector, with businesses donating a total of 10 trillion won, or around $8.4 billion, to strengthen the nation’s biopharmaceutical infrastructure.
SaaS solutions become indispensable at this point since the amount of development and ambition inevitably increases operational complexity.
If you want to read about Key Trends and Insights of SaaS Development in 2025- Click Here!
SaaS platforms provide cloud-based applications that you can access on any device with an internet connection. They have affected several sectors worldwide, particularly the biological sciences. These platforms have become essential to the South Korean Biopharmaceutical sector because they are safe, scalable, and capable of handling massive volumes of data. They also offer tools for adhering to regulations, powerful data analysis and quick communication.
Lets read the need for SaaS Platforms for South Korean Biopharmaceutical Companies in upclose:

SaaS enables biopharmaceutical companies to adapt to changing demands, which is a necessary quality. When it comes to handling an increase in clinical trial data or scaling up production, SaaS platforms are more flexible and cost-effective than traditional IT systems.
Particularly in areas like nanomedicine and precision medicine, the process of creating new medications is intricate and demands efficient management. SaaS systems support by reducing costs, automating jobs, and integrating different domains—such as marketing, supply chain, and clinical trials—into a single system.
In the strictly regulated biopharmaceutical sector, adherence to national and international regulations is necessary. Managing compliance is a difficult task because of South Korea’s rules and the FDA’s stringent criteria for medication development. Scalable SaaS platforms, which automatically provide updates on new regulations, can help comply with standards and facilitate regulatory reporting.
This is important for businesses manufacturing biopharmaceuticals or biosimilars with a view toward international markets. One of the leading biosimilars companies, Samsung Bioepis, must contend with intricate restrictions across many jurisdictions. With the help of SaaS systems, they can maintain compliance and concentrate on innovation and international market expansion.
SaaS’s flexibility to manage vast amounts of data is one of its main advantages. Precision medicine, microbiome research, and cell treatments are becoming more and more popular in South Korea; these industries generate massive volumes of data that require rapid processing. Businesses may obtain valuable insights more quickly by using SaaS solutions to handle large amounts of data.
For example, JW Pharmaceutical and Kolmar Korea develop novel medicine delivery techniques and biosimilars using complex data analysis. SaaS systems facilitate the integration of data from many sources and provide powerful tools to enable businesses to make data-driven choices more effectively.
The biopharmaceutical company benefits greatly from multinational partnerships, suppliers, and research teams working together. Because they offer centralized access to communication tools and project data, scalable SaaS systems facilitate smooth cooperation. This is particularly significant in the biotech ecosystem of South Korea, where a large portion of innovation is driven by collaborations between business, government, and academia.
Collaboration is essential to the government’s “Bio Economy 2.0” agenda, and SaaS platforms are essential for maintaining connections between various parties. As the emphasis on patient-centered medication development increases, virtual clinical trials are becoming more and more common. Platforms such as Medidata have already proven their ability to help in these studies.
IT systems and software that are installed on-site are expensive to grow and maintain. Subscription models are used by SaaS platforms, allowing businesses to pay only for the services they use and lowering startup expenses. Also, companies may avoid paying for internal IT people because SaaS providers handle automatic upgrades and maintenance.
SaaS systems offer a subscription-based business model that enables businesses to expand without requiring significant upfront investment. This is particularly critical for South Korean biotech companies and new businesses, as they frequently have little funding while attempting to develop. These businesses no longer have to invest much in IT infrastructure since SaaS provides them with access to innovative solutions.
Lets look into inspiring real life stories of South Korean Biopharmaceutical Companies that u sedScalable SaaS Platforms and its results in details:
Celltrion, a leader in biosimilars and biopharmaceuticals, struggled with handling large amounts of clinical trial data and regulatory filings. To tackle these challenges, Celltrion introduced a cloud-based platform for managing clinical trials (CTMS) that combined data collection, monitoring, and reporting.

Celltrion was able to expedite their product development process by about 25% by using the CTMS to decrease clinical trial timeframes. Over a two-year period, 100% of new medication application submissions were successful due to the platform’s convenience of regulatory compliance. Furthermore, the system’s increased efficiency and increased data handling resulted in an estimated 20% reduction in overall trial expenses.
One of the biggest contract development and manufacturing organizations (CDMOs) in the world, Samsung Biologics, realised it needed a scalable system for managing its vast activities spread across several sites. The business implemented an enterprise resource planning (ERP) system built on the cloud customized for the biopharmaceutical industry. Real-time data access and departmental interaction were made possible by this platform.

After implementing a new system, Samsung Biologics increased its operating efficiency by 30% in the first year. Also this solution increased data accuracy by 40%, which significantly reduced mistakes in inventory and production management. Furthermore, the cloud-based approach made it simple to scale, which helped the business meet rising demand—particularly when vaccine manufacturing surged during the COVID-19 epidemic.
As Hanmi Pharmaceuticals entered other markets, it ran into a number of challenging regulatory environments. Managing these rules by hand required a lot of effort and was prone to mistakes, which delayed the approval of items.
Hanmi Pharmaceuticals started using MasterControl and TrackWise Digital from Sparta Systems as software to handle regulatory compliance. These technologies help Hanmi in automating its quality control procedures, lowering human mistake rates, and adhering to local and worldwide legal requirements.

Hanmi Pharmaceuticals reduced compliance risks by over 50% and compliance-related delays by 30% by using SaaS for regulatory administration. The automated features simplified regulatory filings, facilitating Hanmi’s more successful entry into new markets.
As 2025 approaches, a number of new developments in the biopharmaceutical industry are closely related to digital transformation, such as:
South Korean biopharmaceutical companies need to use scalable SaaS platforms; it’s essential for them. The complicated process of developing drugs, along with the difficulties of meeting regulations, competing in global markets, and handling large amounts of data, means that digital transformation is unavoidable.
Scalable SaaS platforms will be essential for South Korean enterprises to become leaders in the biotech sector by 2025 and beyond, because to government support such as the “Bio Economy 2.0” initiative and the rapid advancement of biopharmaceutical technology.
Prominent figures like Lee Kun-Hee, the former chairman of Samsung, once said, “Change everything except your wife and children.” This sentiment applies aptly to South Korea’s biopharmaceutical sector, which must embrace digital tools like SaaS to navigate the fast-paced changes of the global market.
In summary, SaaS platforms will be vital for South Korean Biopharmaceutical companies in 2025 as they negotiate a more complicated environment that includes fast technology breakthroughs and stringent regulations. These platforms help businesses improve cooperation and optimize operations by providing the flexibility, efficiency, and data management capabilities required for innovation.
South Korean biopharmaceutical companies can better position themselves to compete internationally and satisfy the industry’s expanding expectations by using scalable solutions. See Yeasitech for further details on how scalable SaaS systems might help your business.
For more information on how scalable SaaS platforms can benefit your organization, you can reach out to Yeasitech.
The biopharmaceutical market is thought to be worth around $300 billion at the moment.
Businesses in the biopharmaceutical sector are faced with a growing number of regulations, intricate data management challenges, and a pressing need for quick innovation. Scalable SaaS platforms facilitate better cooperation, simplify operations, and comply to industry standards.
Personalized health, growing digitization, and a focus on remote work are just a few trends that emphasize the need for flexible, cloud-based solutions that can support changing business models and encourage innovation.
Data management is very important in biopharmaceuticals because it helps with accurate research, meeting regulations, and making good decisions. Scalable SaaS platforms offer strong data analytics and storage solutions, enabling companies to use insights for better results.
YeasiTech is a trusted IT service partner with 8+ years of experience, empowering 250+ businesses with scalable web, mobile and AI solutions.
Explore related topics to broaden your understanding and gain actionable insights that can transform your strategies.